Abstract
Introduction:
The availability of reliable and sensitive methods to assess treatment response is increasingly important in an era of more effective yet costly therapies for patients with Multiple Myeloma (MM). Measurable residual disease (MRD) testing has recently been added to the response evaluation criteria for MM, with NCCN® and IMWG guidelines recommending MRD testing using standardized assays capable of detecting malignant cells at a level of at least 1 in 100,000 cells (1x10-5 sensitivity level). Newer technologies for evaluating MRD testing have demonstrated the ability to detect malignant cells at more sensitive levels (1x10-6) than flow cytometry, conventionally sensitive to 1x10-4 from reference labs. Different levels of sensitivity all beneath the limits of flow cytometry have been shown to correlate with differences in outcome for patients with MM. Therefore, accurate MRD assessment has important implications for patient prognosis and management. Our objective was to evaluate the impact of next-generation sequencing (NGS MRD) (clonoSEQ®) versus traditional flow cytometry techniques (flow) on the number of MM patients with accurate MRD categorization in the United States.
Methods:
We developed a population model to estimate the number of newly diagnosed MM patients accurately assessed for MRD status over a 1-year time horizon using data from SEER, U.S. census, clinical studies, NCCN guidelines, and expert opinion. We used SEER and U.S. census data to estimate the number of newly diagnosed MM patients expected in 2017. We used NCCN guidelines, a recently completed clinical trial (IFM 2009 trial), publicly available literature and expert opinion to estimate the timing, treatment sequence, and response evaluation time points in the first year after initial diagnosis including the induction, consolidation, stem cell transplant, and maintenance treatment phases. We used the IFM study and published literature to estimate the proportion of patients expected to transition to each treatment phase. We used clinical studies (IFM) comparing the test characteristics of NGS MRD vs. flow to estimate the comparative accuracy of the tests, using NGS MRD as the gold standard for accurate categorization. Finally, we evaluated three market penetration levels in the first year, 25%, 50%, 100%. The primary model outcome was the number of accurately categorized patients in the United States over a one year timeframe.
Results:
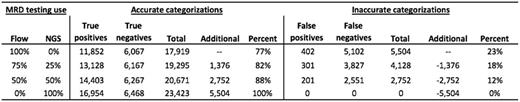
We found that in 1-year, 17,919 (77% accurate with 11,852 True positive and 6,067 True negative) MM patients would be accurately categorized and 5,504 would be inaccurately categorized (402 false positives and 5,102 false negatives) using flow alone. If NGS MRD was used for 25%, 50%, or 100% of the testing, an additional 1,376, 2,752, or 5,504 patients would be appropriately categorized, respectively.
Conclusions:
Our analysis demonstrates that MRD with NGS with significantly improved depth of measurement could substantially increase the number of patients appropriately assessed for MRD status in the U.S. With clinical decisions, e.g. treatment switching, treatment continuation, and use of SCT, increasingly being tied to MRD evaluation, obtaining accurate MRD status will have important clinical and economic implications for patients and health systems.
Zimmermann: Adaptive Biotechnology: Consultancy. Kirsch: Adaptive Biotechnology: Employment. Carlson: Adaptive Biotechnology: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal